
The groups numbered in the North American system are the two "tall" columns on the left side of the "dip" in the chart, as well as the six "tall" columns to the right of it. Both versions of the periodic table show seven periods. In particular, the North American system numbers only eight groups, leaving 10 columns unnumbered, whereas the other system -approved by the International Union of Pure and Applied Chemistry (IUPAC) -numbers all 18 columns. The periodic table is examined in depth within the essay devoted to that subject, and among the specifics discussed in that essay are the differing systems used for periodic-table charts in North America and the rest of the world. For one thing, it makes it possible to see at a glance families of elements, many of which either belong to the same group (column) or the same period (row) on the table. Certainly other organizational systems exist, but Mendeleev's table is the most widely used -and with good reason. HOW IT WORKS The Basics of the Periodic TableĬreated in 1869, and modified several times since then, the periodic table of the elements developed by Russian chemist Dmitri Ivanovitch Mendeleev (1834-1907) provides a highly useful means of organizing the elements. The nonmetals form a loosely defined cross-family grouping, as do the metalloids. Families on the periodic table include, in addition to noble gases and halogens, the alkali metals, alkaline earth metals, transition metals, lanthanides, and actinides. Despite these apparent differences, common electron configurations identify the halogens as a family. Fluorine is a member of another family, the halogens, which have so many shared characteristics that they are grouped together, despite the fact that two are gases, two are solids, and one -bromine -is one of only two elements that appears at room temperature as a solid.
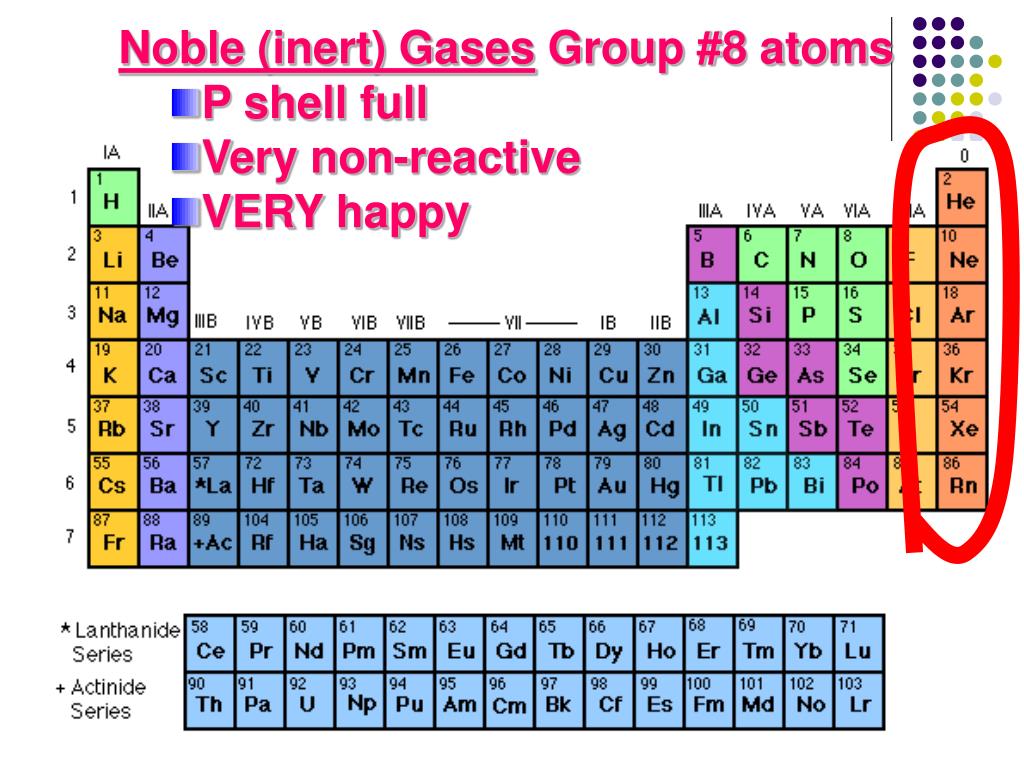
All noble gases, for instance, tend to be highly nonreactive: only a few of them combine with other elements, and then only with fluorine, the most reactive of all substances.
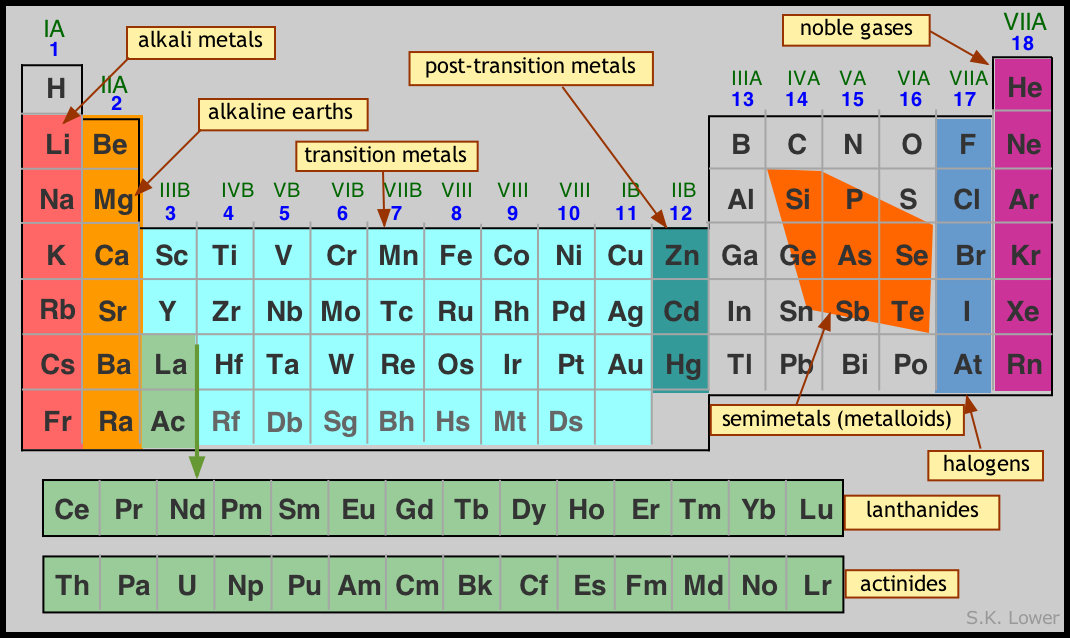
The term "family" is used to describe elements that share certain characteristics -not only in terms of observable behavior, but also with regard to atomic structure.


 0 kommentar(er)
0 kommentar(er)
